stève consultants is your partner to obtain or maintain reimbursement of your products on the French market (medicinal products, vaccines, medical devices, in vitro assay).
We have experience in many therapeutic areas notably in oncology, immunology, infectious diseases (hepatitis C, HIV, etc.) or in rare diseases.
Our recognized talents bring a multifunctional expertise given their background and variety of experience (health authorities, pharmaceutical industry, hospital and academic settings, etc.)
2006
consultants
loyal customers
Stève Bénard – Executive manager
Josselin Boussel – Head of Market access
For 15 years, stève consultants has been specialized in:
- Price / reimbursement strategy consulting for health products
- Developing and valorizing scientific and medico-economic evidences in line with health authorities requirements (HAS and CEPS), possibly with the support of recognized experts
- Writing and submission of reimbursement dossiers (early access, transparency, CNEDiMTS and economic dossiers)
- Customer support throughout the dossier evaluation by health authorities
Asset: Our recognized experience on the latest innovations (CAR-T, immunotherapy, gene therapy, targeted therapy…) and orphan drugs
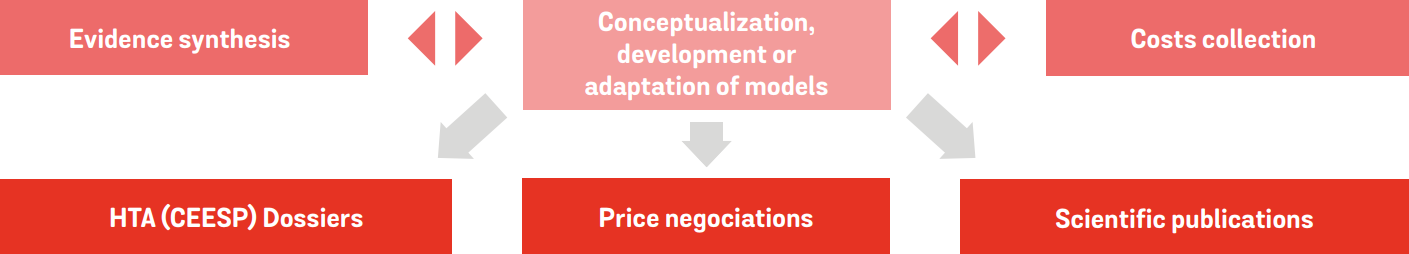
Our expert team will support you in:
-
Development or adaptation of health economic models (CE, BI models… )
-
Development of HTA dossiers (CEESP/HAS)
-
Development of simulation tools to support medicomarketing strategy
-
Data collection and cost studies
-
Evidence synthesis (meta-analysis, indirect comparison, and post-hoc analyses)
Lucile Marié – HEOR Director
Françoise Bugnard – RWE Director
Our dedicated team brings its expertise to support your realworld evidence generation strategy leveraging clinical and medico-administrative databases from the French Health Data Hub (SNDS). We conduct:
-
Pharmaco-epidemiology studies (descriptive epidemiology, target population, effectiveness and real-world safety…)
-
Healthcare management studies (treatment patterns, healthcare clusters, off-label/appropriate use…)
-
Health economic studies (healthcare resource use, burden of disease…)







